On the morning of March, 6th in 2021, foreign teachers and administrators of International Exchanges Office in Shangqiu Normal University got the Novel Coronavirus vaccinations voluntarily, meanwhile, everyone agreed and signed the informed consents.

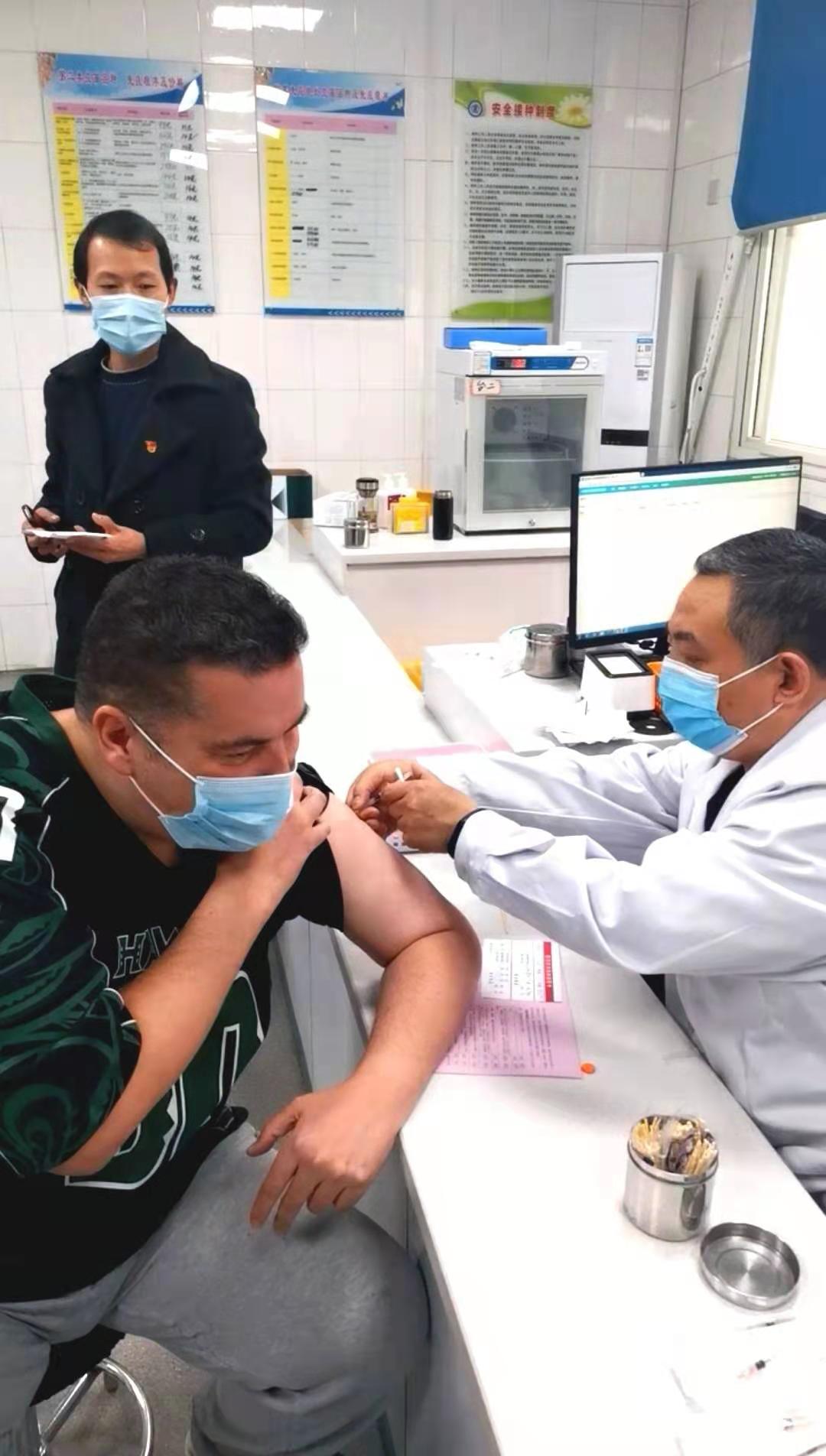
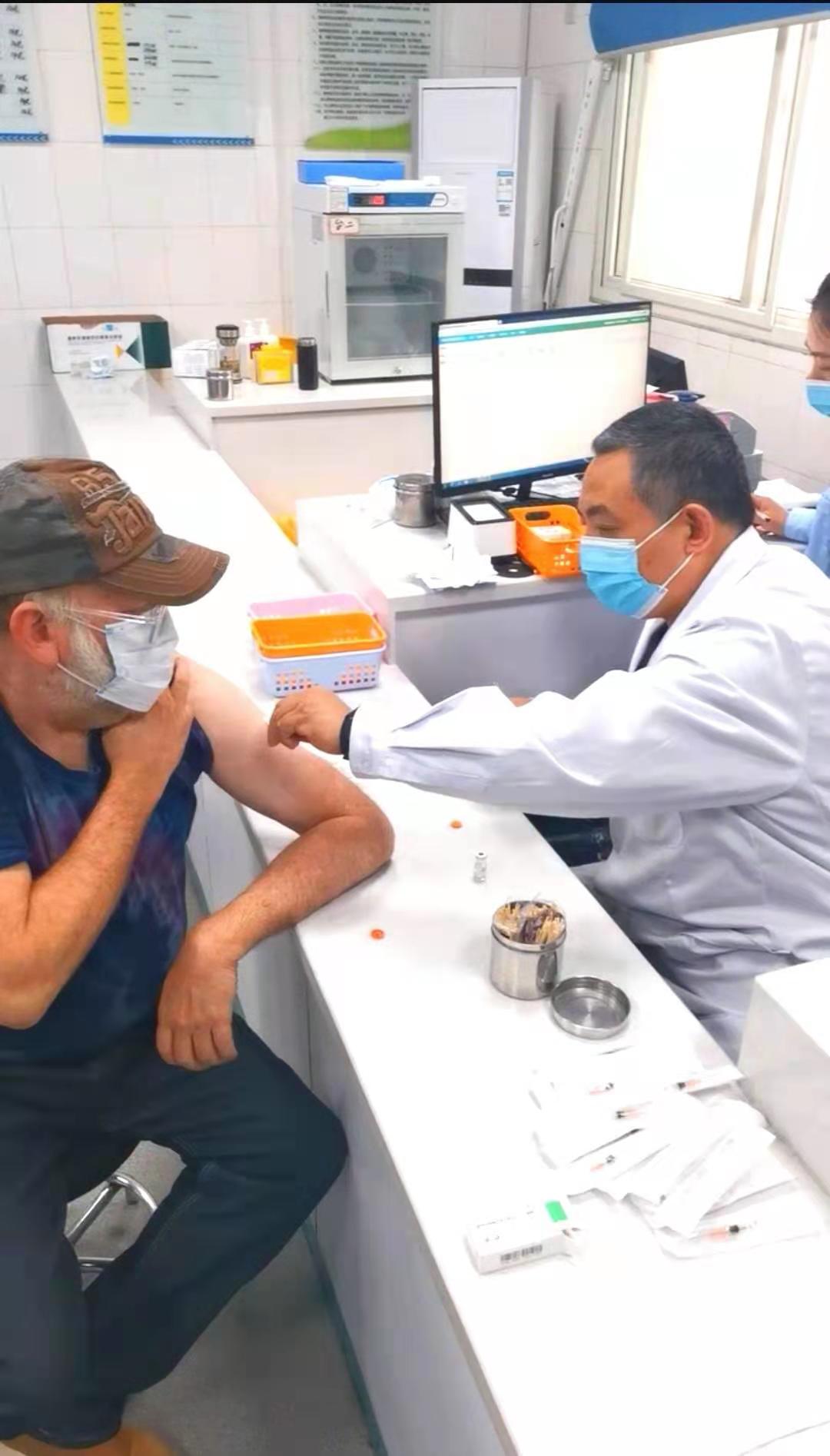
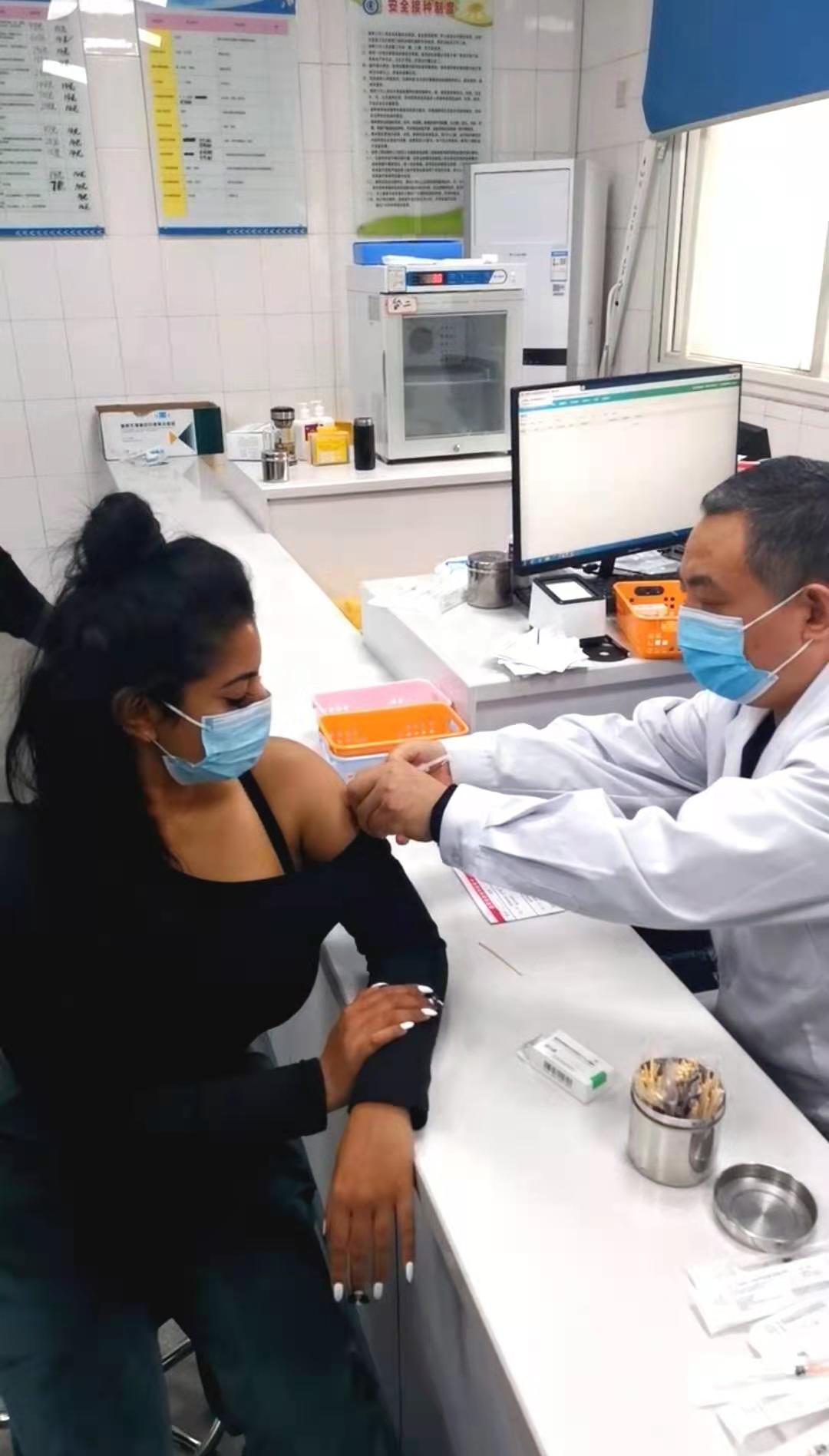
About vaccinations:
【Vaccine】The novel coronavirus vaccine, the tolerable dose is 0.5ml each person.
【Vaccine Effect】Vaccine clinical trial data showed that the ratio of moderate antibody production in the first two weeks of total immunity was 95.76%, based on the criteria of the antibody titration≥1:4. Based on the clinical study data for the completed vaccine immunogenicity and safety, suggesting that the vaccine may have potential clinical application value, however since the study on the protective effect of new coronary pneumonia has not been completed, the vaccine is not yet certain to have protective effect on new coronary pneumonia.
【Object of Vaccination】The susceptible people of novel coronavirus must be 18 to 59 years old.
【Immunization Program and Approach】Intramuscular injection of upper deltoid muscle. Two doses were inoculated at an interval of 14-28 days.
【Adverse Reactions】The clinical trial data showed that the highest incidence of adverse reaction was pain at the site of vaccine inoculation within 0 to 28 days after vaccination, with a rate of 19.35%.The second was fatigue, the incidence rate was 5.91%.The rate of other main incidence was ≥1%, such as: diarrhea, headache, fever, muscle pain, nausea, cough, vaccination site swelling, redness, etc. There were no serious adverse reactions.
In addition, previous animal studies on other coronavirus vaccines have shown that the disease caused by the virus worsens when re-infected with the virus after inoculation with the inactivated vaccine. Although this vaccine has not been observed in large animal trials and phase I/II human clinical trials, it is not clear whether this vaccine also has the above-mentioned safety problems due to the completion of phase III clinical studies.
【Inoculation of Taboo】Due to limited clinical trial data, the following populations were excluded from this vaccination. Please clarify whether the following conditions exist:
1. Ages are not 18 to 59. |
yes |
no |
2. Pregnancy. |
yes |
no |
3. Severe allergic reactions from previous vaccination have occurred.(such as acute anaphylaxis, urticaria, skin eczema, dyspnea, angioneurotic edema, or abdominal pain) |
yes |
no |
4. History or family history of convulsions, epilepsy, encephalopathy or mental illness. |
yes |
no |
5. Severe liver and kidney diseases, uncontrolled hypertension (systolic blood pressure ≥140mmHg, diastolic blood pressure ≥90mmHg), diabetes complications, malignant tumors, various acute diseases or acute onset of chronic diseases. |
yes |
no |
6. Has been diagnosed with congenital or acquired immunodeficiency, HIV infection, lymphoma, leukemia or other autoimmune disease. |
yes |
no |
7. Known or suspected medical conditions include: severe respiratory disease, severe cardiovascular disease, liver and kidney disease, and malignant tumor. |
yes |
no |
8. Previous participation in vaccine clinical studies or vaccination of novel coronavirus vaccine. |
yes |
no |
【Attention】1. After vaccination, stay on site for 30 minutes before leaving.
2. The protection standard shall not be lowered after inoculation.
